Water Quality Parameters: pH
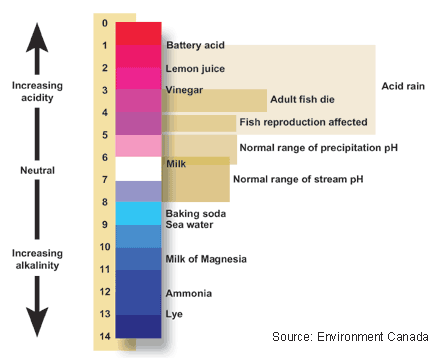
Figure 4.8: The pH scale indicates the acidity or alkalinity (basic-ness) of a solution.
Image from URL: http://ga.water.usgs.gov/edu/phdiagram.html
Backing up to the previous section, let’s recall what makes up water. Two charged particles: H+ and OH- are what make up pure water.
Remember that water in the environment is never pure; it is an aqueous solution containing many dissolved salts and even gases. The pH of a solution is a measure of the amount or concentration of the H+ ions in a solution, which tells us if our water solution is an acid or a base. Some important things to know about pH:
- It is measured in standard units on a logarithmic scale ranging from 0 to 14. Logarithmic means that measurements on the scale increase or decrease by a power of 10.
- Absolutely pure water, or a solution that is neither acidic or basic measures a 7, or right in the middle of the scale. We call solutions with a pH of 7 “neutral.”
- If there are more H+ ions in solution than OH-, the pH will be less than 7, or acidic.
- If there are more OH- ions than H+, the solution will be above 7 or basic.
- A pH of 4 is 100 times more acidic than a pH of 6 and 1,000 time more acidic than a pH of 7, and so on.
- The pH of healthy, natural streams and rivers in Montana fall anywhere between 6.5 and 8.5, meaning they can be slightly acidic or basic, or “near nuetral” as these pH values are also referred. Any stream or water source with a measurement outside of this range (either more acidic or basic) indicates that some human impact (e.g. pollution) or natural process is changing the chemical state of the water. For example, runoff from abandoned mines or mining wastes high in iron pyrite can significantly lower the pH of a stream. This process, facilitated by the oxidation of the pyrite which releases sulfuric acid into the environment, is known as Acid Rock or Mine Drainage. Conversely, streams that are fed by groundwater in limestone geology tend to have a higher or basic pH. This phenomenon is caused by the geologic dissolving of CaCO3 into water which results in formation of bicarbonate, a basic or alkaline substance.