Structure & Properties of Water
The noblest of the elements is water.
-Pindar, Greek Poet, 476 B.C.
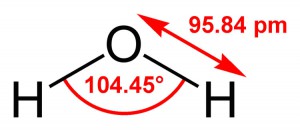
Figure 4.2: The bond arrangement of a water molecule, including the bond angles and bond distances.
Image from URL: http://en.wikipedia.org/wiki/Water_(molecule)
Of course, water is not an element, and almost everyone knows the chemical formula for the compound water: H2O.
Many also know its bend arrangement, the atoms forming the H-O-H bonds. In Figure 1.1, both the bond angles and bond distances are represented. Molecular distances may at first seem confusing, but they are becoming more common in everyday language, e.g. use of the word nanosecond or nano-technology.
One picometer (pm) equals 10-12 meters (m). That is, you would have to write a decimal point followed by 11 zeros before you could write the “1”. Most of the world uses the meter as the standard for distance measurement (click here to read about the International System of Units, or SI); molecular-scale distances are expressed as picometers, nanometers (nm; 1 nm = 10-9 m), or in older literature, as Ångströms (Å; 1 Å = 10-10 m). Therefore the hydrogen-oxygen bond distance would be 95.84 pm, as indicated in the figure below, and it could also be written as 0.9584 Å, or 0.009584 nm.
Water is frequently referred to as the “Universal Solvent” because of the large number of compounds that will dissolve in it. Many common products we consume are aqueous solutions (eye drops, IV solutions, soda, power drinks, etc).