Water Phase Change
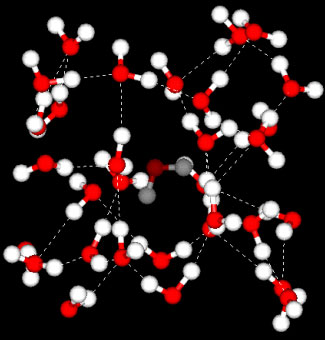
Figure 5.6: Liquid water (at left) can be thought of as a seething mass of H2O molecules in which hydrogen-bonded clusters are continually forming, breaking apart, and re-forming. The more crowded and jumbled arrangement in liquid water can be sustained only by the greater amount thermal energy available above the freezing point (0°C).
Images from URL:http://ssrl.slac.stanford.edu/nilssongroup/pages/project_liquid_structure.html
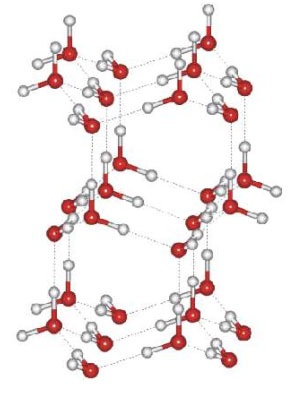
Notice the greater openness of the ice structure (at right). This is necessary to ensure the strongest degree of hydrogen bonding in a uniform, extended crystal lattice.
Images from URL:http://ssrl.slac.stanford.edu/nilssongroup/pages/project_liquid_structure.html
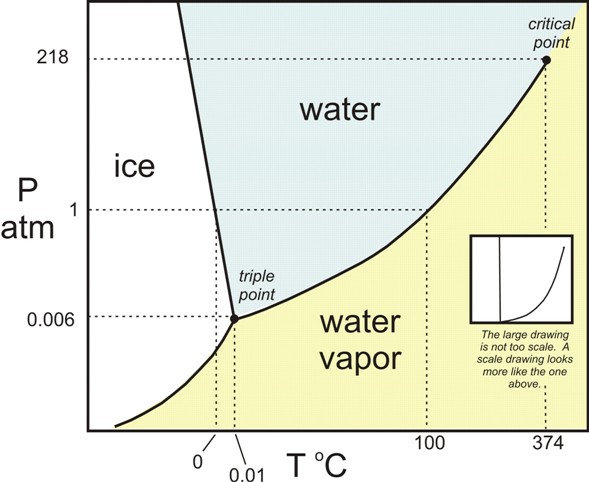
A phase change is a change from one state to another without a change in chemical composition. These changes are induced by the effects of temperature and/or pressure:
The transitions are:
- Solid-to-liquid transition – melting
- Liquid-to-solid transition – freezing
- Liquid-to-gas transition – evaporation
- Gas-to-liquid transition – condensation
- Solid-to-gas transition – sublimation
- Gas-to-solid transition – deposition
Download the Measuring the Temperature of Water, Snow and Ice document (pdf) for a variety of activities for students of various ages for measuring the temperature of snow and water. You can also view Measuring the Temperature of Water in the Content Map at the left.